What Is The Z Effective Of Sodium . We need to talk of effective nuclear charge. At r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. These two factors are important determinants in shielding (see next section), and they are used to calculate a shielding constant (σ) used in. S = average amount of. Z = denotes the number of protons existing in the nucleus. Zeff = the effective nuclear charge. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the. We denote it by zeff. For the first electron around the nucleus, the effective nuclear. At intermediate values of r, the.
from www.findatopdoc.com
Z = denotes the number of protons existing in the nucleus. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the. We need to talk of effective nuclear charge. We denote it by zeff. At r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. Zeff = the effective nuclear charge. For the first electron around the nucleus, the effective nuclear. These two factors are important determinants in shielding (see next section), and they are used to calculate a shielding constant (σ) used in. At intermediate values of r, the. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the.
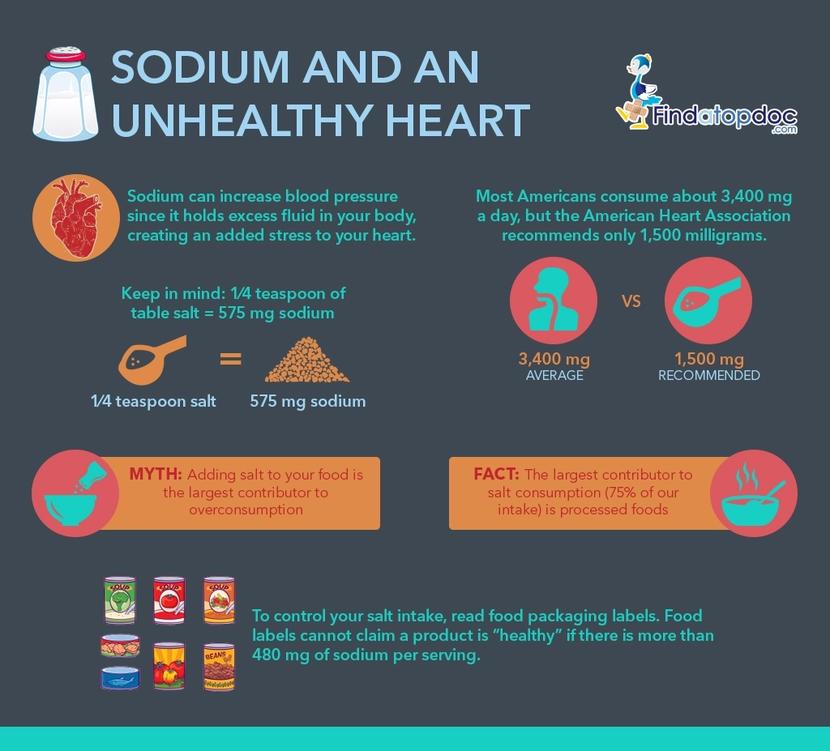
How to Control Your Sodium Levels FindaTopDoc
What Is The Z Effective Of Sodium The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the. S = average amount of. Zeff = the effective nuclear charge. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the. Z = denotes the number of protons existing in the nucleus. We need to talk of effective nuclear charge. For the first electron around the nucleus, the effective nuclear. At r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. At intermediate values of r, the. These two factors are important determinants in shielding (see next section), and they are used to calculate a shielding constant (σ) used in. We denote it by zeff.
From www.heart.org
The Salty 6 Foods Highest in Sodium Infographic American Heart What Is The Z Effective Of Sodium Z = denotes the number of protons existing in the nucleus. These two factors are important determinants in shielding (see next section), and they are used to calculate a shielding constant (σ) used in. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the. The effective nuclear charge may be defined as the actual nuclear charge (z). What Is The Z Effective Of Sodium.
From www.youtube.com
Hypothesis z test sodium example YouTube What Is The Z Effective Of Sodium We denote it by zeff. Zeff = the effective nuclear charge. At r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the. For the first electron around the nucleus, the effective nuclear. At intermediate values of r,. What Is The Z Effective Of Sodium.
From www.numerade.com
SOLVED 'Reaction of sodium dichromate with potassium iodide in What Is The Z Effective Of Sodium For the first electron around the nucleus, the effective nuclear. These two factors are important determinants in shielding (see next section), and they are used to calculate a shielding constant (σ) used in. S = average amount of. Zeff = the effective nuclear charge. We denote it by zeff. At r ≈ 0, the positive charge experienced by an electron. What Is The Z Effective Of Sodium.
From www.youtube.com
The `Z_(eff)` for 3d electron of `Cr` `4s` electron of `Cr` `3d What Is The Z Effective Of Sodium What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the. Zeff = the effective nuclear charge. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the. S = average amount of. We need to talk of effective nuclear charge. These two factors are. What Is The Z Effective Of Sodium.
From www.medchunk.com
Is Sodium 138 normal, high or low? What does Sodium level 138 mean? What Is The Z Effective Of Sodium We denote it by zeff. At intermediate values of r, the. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the. Zeff = the effective nuclear charge. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the. Z = denotes the number of. What Is The Z Effective Of Sodium.
From www.chegg.com
Solved 5. Using the table below of Zefi, calculate the first What Is The Z Effective Of Sodium Z = denotes the number of protons existing in the nucleus. For the first electron around the nucleus, the effective nuclear. At r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. These two factors are important determinants in shielding (see next section), and they are used to calculate a. What Is The Z Effective Of Sodium.
From webapi.bu.edu
💋 Why is sodium where it is on the periodic table. Where is sodium on What Is The Z Effective Of Sodium Z = denotes the number of protons existing in the nucleus. For the first electron around the nucleus, the effective nuclear. At intermediate values of r, the. We denote it by zeff. We need to talk of effective nuclear charge. Zeff = the effective nuclear charge. At r ≈ 0, the positive charge experienced by an electron is approximately the. What Is The Z Effective Of Sodium.
From www.nhlbi.nih.gov
Choose Foods Low in Sodium NHLBI, NIH What Is The Z Effective Of Sodium At r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. We denote it by zeff. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the. We need to talk of effective nuclear charge. For the. What Is The Z Effective Of Sodium.
From www.indianchemistry.com
Understanding z Effective Trends in the Periodic Table and Its What Is The Z Effective Of Sodium These two factors are important determinants in shielding (see next section), and they are used to calculate a shielding constant (σ) used in. For the first electron around the nucleus, the effective nuclear. At intermediate values of r, the. S = average amount of. We need to talk of effective nuclear charge. We denote it by zeff. Z = denotes. What Is The Z Effective Of Sodium.
From mmaawarrhitam.blogspot.com
As Zeff Increases The Electron Experiencing That Zeff Will / Periodic What Is The Z Effective Of Sodium These two factors are important determinants in shielding (see next section), and they are used to calculate a shielding constant (σ) used in. Zeff = the effective nuclear charge. Z = denotes the number of protons existing in the nucleus. We need to talk of effective nuclear charge. At intermediate values of r, the. S = average amount of. What. What Is The Z Effective Of Sodium.
From www.youtuberandom.com
How To Use Slater's Rule to Estimate The Effective Nuclear Cha... What Is The Z Effective Of Sodium We denote it by zeff. S = average amount of. Zeff = the effective nuclear charge. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the. At intermediate values of r, the. We need to talk of effective nuclear charge. For the first electron around the nucleus, the effective nuclear. The effective nuclear charge may be defined. What Is The Z Effective Of Sodium.
From www.horiba.com
Determination of Sodium and Salt Content in Food Samples HORIBA What Is The Z Effective Of Sodium What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the. We need to talk of effective nuclear charge. Z = denotes the number of protons existing in the nucleus. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the. For the first electron. What Is The Z Effective Of Sodium.
From diet2nourish.com
Get Complete Guide to LowSodium Diet Plan Diet Blogs By Dt. Priyanka What Is The Z Effective Of Sodium We denote it by zeff. These two factors are important determinants in shielding (see next section), and they are used to calculate a shielding constant (σ) used in. At intermediate values of r, the. At r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. The effective nuclear charge may. What Is The Z Effective Of Sodium.
From www.breakingatom.com
Nuclear Charge What Is The Z Effective Of Sodium These two factors are important determinants in shielding (see next section), and they are used to calculate a shielding constant (σ) used in. For the first electron around the nucleus, the effective nuclear. Z = denotes the number of protons existing in the nucleus. Zeff = the effective nuclear charge. At r ≈ 0, the positive charge experienced by an. What Is The Z Effective Of Sodium.
From byjus.com
14. If Zeffective of sodium is x then Z effective of carbon is a)x +.90 What Is The Z Effective Of Sodium These two factors are important determinants in shielding (see next section), and they are used to calculate a shielding constant (σ) used in. At r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. Zeff = the effective nuclear charge. We need to talk of effective nuclear charge. What is. What Is The Z Effective Of Sodium.
From www.dreamstime.com
Sodium stock illustration. Illustration of background 83614893 What Is The Z Effective Of Sodium The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the. Z = denotes the number of protons existing in the nucleus. What is the effective attraction \(z_{eff}\) experienced by the valence electrons in the. At intermediate values of r, the. At r ≈ 0, the positive. What Is The Z Effective Of Sodium.
From www.findatopdoc.com
How to Control Your Sodium Levels FindaTopDoc What Is The Z Effective Of Sodium We need to talk of effective nuclear charge. S = average amount of. Z = denotes the number of protons existing in the nucleus. These two factors are important determinants in shielding (see next section), and they are used to calculate a shielding constant (σ) used in. At r ≈ 0, the positive charge experienced by an electron is approximately. What Is The Z Effective Of Sodium.
From slidesharenow.blogspot.com
How To Calculate Z Effective slideshare What Is The Z Effective Of Sodium We denote it by zeff. At intermediate values of r, the. These two factors are important determinants in shielding (see next section), and they are used to calculate a shielding constant (σ) used in. At r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. We need to talk of. What Is The Z Effective Of Sodium.